L. Buono, A. D'Amore, G. Giuliani, F. Polonara
Dipartimento di Energetica, Università di Ancona
60100 Ancona, Italia
Introduction
The rapid freezing of food
stuffs is a process that simultaneously guarantees a better product quality
and a greater productivity for the food industry. An important factor to ensure
rapid freezing is to work with very low temperatures of the refrigerant fluid:
the use of liquid nitrogen in such applications (normal boiling temperature
-196°C) is a classic, but it carries the drawback that using a fluid at
nearly -200°C to cool a product to -18°C is an obvious thermodynamic
absurdity. The same efficacy as liquid nitrogen, in terms of freezing rate,
can be obtained by using the refrigerant fluid at temperatures of around -75°C:
given that, in the majority of applications, the final vehicle for transferring
the heat energy to the product is air, the option of the reversed Joule-Brayton
cycle, in which the working fluid is air, is of considerable interest.
With this in mind, a research project coordinated by TecnoMarche (the scientific
and technological park of the Marche region in Italy) was organized with a view
to designing, implementing and testing a system for freezing ready-to-eat foods
comprising a revolving drum freezer operating with air at -75°C produced
by a refrigerator operating on an air cycle.
This paper describes the prototypes of freezer chamber and refrigerator that
have just been constructed and the simulation techniques used to optimize the
freezing process.
Prototype of refrigerator operating on an air cycle
The reversed gas, or Joule-Brayton
cycle has been used extensively in the past for refrigeration applications wherever
the fundamental requirements included the compact dimensions of the equipment
(for instance, for air conditioning on board aircraft). This system is far less
efficient than the steam compression cycle, however, and this fact prevents
its genuinely widespread use.
The loss of efficiency with the reversed gas cycle by comparison with the steam
compression cycle diminishes progressively, however, with lower temperatures
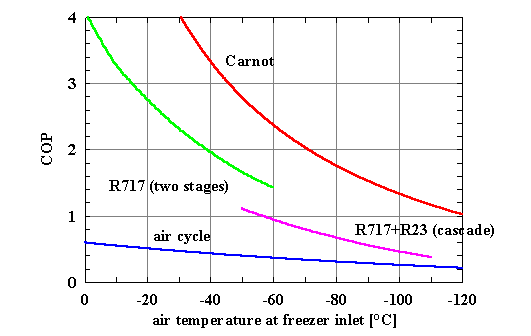
of the cold source, as shown in the analysis in Figure 1.

Figure 1 - Trend of the
efficiency of different types of reversed cycle
(hot source temperature =30°C, vapour compression cycles: DT exchangers
=10 K, efficiency of the air cycle: first-stage compressor =0.80,
second-stage compressor =0.75, turbine=0.82)
The refrigerator, made using
Japanese technology (AIRS, Kajima Corp.), operates on a reversed Joule-Brayton
double-compression air cycle with intermediate inter-refrigeration and internal
regeneration.
The two compression stages are implemented by two centrifugal compressors, the
second of which is coupled mechanically to the turbine acting as an expansion
organ. The compressor-turbine assembly (bootstrap) is made using European technology
(Atlas-Copco).
Internal regeneration is achieved with an exchanger using a secondary fluid
(silicone oil) that enables an end-of-expansion temperature of -75°C to
be achieved.
The refrigeration cycle is interrupted as necessary by a defrosting cycle, in
which the regeneration cycle and the freezer are disabled, while the turbine
is bypassed.
The machine comprises the following main components (Figure 2):
first-stage compressor
first inter-refrigeration exchanger (water cooled)
second-stage compressor
second inter-refrigeration exchanger (water cooled)
intermediate-fluid heat regenerator (high-pressure side)
turbine
ice trap
drum freezer (external user)
intermediate-fluid heat regenerator (low-pressure side)
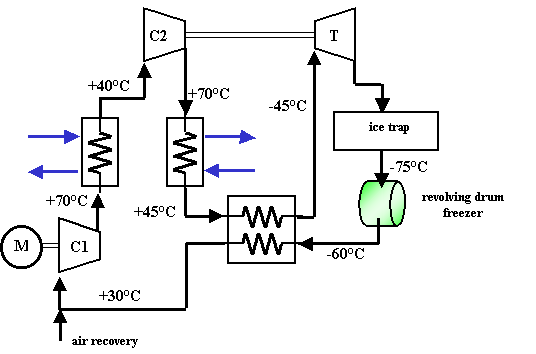
Figure 2 - Block diagram of the AIRS machine operating on an air cycle
The compressor is a classic
centrifugal compressor driven by an electric motor operating at a voltage of
200 Vac, controlled by an inverter with a frequency variable from 50 to 60 Hz
and connected to an overgear so that its rotation can be varied from 30,000
to 36,000 rpm. The various operating stages enable the temperature of -75°C
to be reached gradually.
Once it has been compressed, the hot air is sent to the first heat exchanger
(a finned coil), where it undergoes the first cooling phase by means of water
coming from a dedicated circuit.
From the first exchanger, the air goes to the second compressor, subsequently
passing into a second heat exchanger, which is also a finned coil connected
to the same cooling circuit as the first exchanger.
The temperature of the air is further lowered before it is sent to the turbine,
passing through an intermediate-fluid heat regenerator (high-pressure side).
Once it has left the turbine, and before it is sent to the freezer, the air
is diverted through a snow trap, which intercepts any frost forming in the part
of the circuit at a temperature of less than 0°C; the presence of the snow
trap makes it necessary to provide for a defrosting cycle at high temperatures
so that the build-up of ice can be eliminated fairly quickly.
The air is subsequently sent to the external user through a dedicated circuit.
On leaving the user, before it is sent back to the compressor, the air passes
through the regenerator again (low-pressure side).
As mentioned earlier, the first and second heat exchangers are served by the
same cooling circuit, composed of a recirculating pump on the warm side and
one on the cold side, a plate exchanger and a chiller. The operation of all
the various elements involved (pumps and fans) is governed by the AIRS control
system.
In the case of the present installation, logistic problems made us decide not
to use the chiller, thus also excluding the pump on the cold side and using
the water in the circuit at the industrial plant where the prototype is installed
as the cooling fluid instead.
The intermediate-fluid heat regenerator is composed of two finned batteries
served by a closed circuit comprising a tank containing the intermediate fluid,
which is a silicone oil (given the low temperatures required). The silicone
oil is circulated by means of a suitable pump in the high-pressure regenerator,
where it removes heat from the air in order to restore said heat to the air
in the regenerator on the low-pressure side.
Prototype of revolving drum freezer
The freezer is composed
of central circular body, shaped rather like a drum, that contains the products
to freeze; a flow of cold air is delivered to the drum from the refrigerator.
The air exchanges heat with the products and is discharged through a pipe lying
coaxial to the delivery pipe (Tout=-60°C)
Figure 3 shows a picture of the freezer prototype, with the opening for loading
and unloading the food products at the front, and the delivery and return air
pipes at the back.

Figure 3 - Prototype of revolving drum freezer
The freezer is coupled to an electric motor that enables a rotating movement of the drum, at a speed that can vary continuously between 6 and 30 rpm, so as to prevent the food products from sticking to the inside walls.
Modeling methods used to optimize the freezing process
The aim of fluid thermodynamic
analysis, implemented in this case using CFD (Computational Fluid Dynamics)
techniques, is to obtain a detailed analysis of the heat exchange between the
products and the cold air so as to be able to take action by means of geometrical
modifications to the delivery and/or return piping in the event of any problems
of uneven freezing of the products. It is also important to estimate the time
it takes to freeze the product.
After the fluid thermodynamic analysis, the mathematical model will be validated
by means of a series of measurements at the test bench, using thermocouples
and a hot wire anemometer for point-by-point temperature and velocity measurements
in order to assess any differences with respect to the results obtained with
the calculation procedure.
The fluid thermodynamic analysis was carried out by simplifying the problem.
First of all, a two-dimensional problem of the freezer was considered, disregarding
the relative motion; then we analyzed the case in which the machine was made
to turn at a constant speed; and, finally, the problem was dealt with from a
three-dimensional point of view.
The sequence of steps involved in studying a problem with computational fluid
dynamics is listed below:
analyzing the problem in question in detail, with a view to simplifying
the case considered in the study;
constructing the calculation mesh;
establishing the thermophysical properties of the materials involved;
establishing the initial and boundary conditions;
analyzing the results obtained (temperatures, velocities, pressures, etc.).
First of all, a mesh was created with 9000 cells, represented in Figure 4.
An implicit segregated, non-stationary model was prepared as the solution-finder.
Moreover, the energy equation was enabled and the following were assumed as
the boundary conditions:
INLET: the inlet conditions of the fluid were specified, i.e. temperature
(Tin = -75°C), density (variable with temperature, according to the ideal
gas equation), velocity ((vin= 8 m/s), turbulence conditions (intensity of turbulence
equating to 10% and turbulent mixing length amounting to 0.02 m).
OUTLET: no particular outlet conditions were specified because the simulation
program proceeds directly with the calculation of the various parameters.
The conditions of the WALL were also defined, considering that the walls must
be conductive: the program calls for a separation of the solid and fluid cells
by means of a wall when the heat transmission is enabled.
The decision to study the two-dimensional case initially was dictated mainly
by the calculation times needed by the program's solution-finder. The number
of cells used to schematically represent the calculation domain was also chosen
with the calculation times in mind. To give an idea, we can say that the calculation
time needed for the two-dimensional model is of the order of several hours,
whereas for three-dimensional models, with the number of cells amounting to
around 500,000, the calculation takes several days (and we have to remember
that said times are dead times in the design phase and must consequently be
reduced as far as possible).
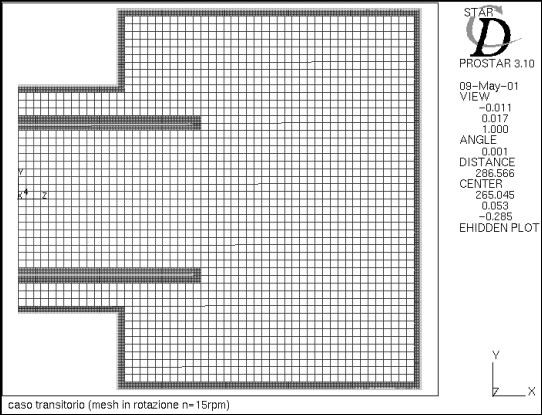
Figure 4 - Calculation mesh
After these essential introductory
considerations, we can go on to provide an overview of the preliminary results
obtained so far, relating to the velocity and temperature profiles, since the
greatest constraint lies in relation to the variation in temperature between
the freezer inlet and outlet.
The figures given below show examples of the temperatures and velocities in
the calculation mesh, without adding any food products, in order to analyze
the thermal flows exchanged between the freezer and the outside environment
first.
The transient case is illustrated (i.e. also simulating the rotation of the
drum), which is more realistic in the case in point. You can follow the evolution
of the flow through the freezer from an initial instant up until such time as
a thermal equilibrium is achieved. From then on, we can assume that the analysis
is complete and verify the results obtained.
Figures 5 and 6 compare the velocity vectors in the calculation mesh at two
different times.
Figure 5 represents the velocity vectors at the time t=0.002 s: in practical
terms, we obtained an image of the moment when the first cold flow arrives in
the freezer and the velocity vectors separate out symmetrically, then leave
through the return pipe.
In Figure 6, at the time t=1.9 s, the flow has transited through the freezer
to depart through the return pipe. A common feature of the cases represented
in the two figures is the reflux of the fluid, which deviates as soon as it
enters the freezer in order to leave through the return pipe. This phenomenon
was confirmed by the fact that the velocity of the fluid tends to diminish progressively
as it comes closer to the freezer's cover. While, on the one hand, the reflux
of cold air represents a hazard for the successful outcome of the product freezing
process, on the other it represents a positive factor for the air cycle, which
requires a fluid return at a temperature no higher than approximately -60°C.
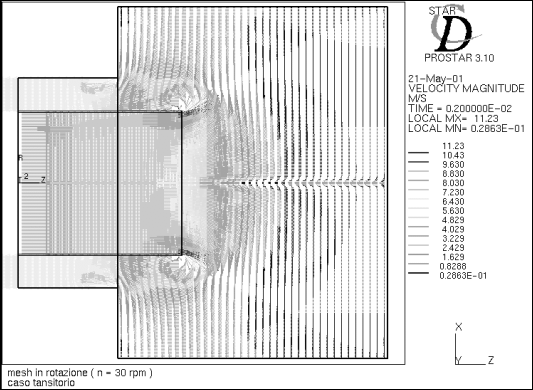
Figure 5 - Velocity vectors at time t=0.002 s

Figure 6 - Velocity vectors at time t=1.9 s
Figures 7 and 8 show the
temperature profiles at two different times.
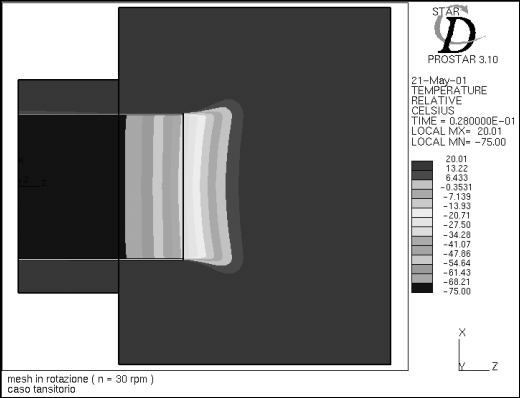
Figure 7 - Temperature profile at time t=0.002 s
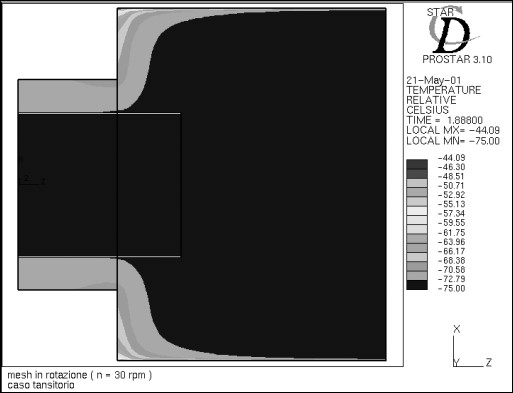
Figure 8 - Temperature profile at time t=1.9 s
In Figure 7 the cold front
advances slowly through the freezer and tends to separate, confirming the reflux
phenomenon already encountered with the velocity vectors.
Figure 8 shows the temperature distribution at the time t=1.88 s. The return
temperature averages around -70°C, while the temperature inside the freezer,
even in the most "critical" areas, never exceeds -44°C.
Conclusions
The next steps in our research
will be oriented towards establishing potential fluid thermodynamic variations
inside the freezer as a result of changes to the geometry of the refrigerated
air intake pipe.
Then various products will be placed on the floor of the freezer to assess the
heat load absorbed and the feasibility of freezing the product with an I.Q.F.
(Individually Quick Frozen) type of treatment.